Ozone is formed in the troposphere when sunlight causes complex photochemical reactions involving oxides of nitrogen (NOx), and VOCs that originate chiefly from gasoline engines and burning of other fossil fuels. Woody vegetation is another major source of VOCs (e.g. isoprene or terpene, formaldehyde, acetaldehyde, methyl‐ethyl‐ketone, acetone, etc.). NOx and VOCs can be transported long distances by regional weather patterns before they react to create ozone in the atmosphere, where it can persist for several weeks. Major photochemical reactions that cause ozone formation are given in the following.
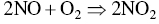
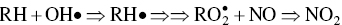
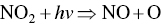
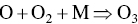
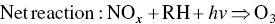
Visibility and Regional Haze
The oxidizing agents, ozone (O3), PAN, peroxybenzoyl nitrate, and other trace substances react with nitrogen oxides and VOCs and produce smog. These components of photochemical smog which are most damaging to plants and detrimental to human health are these photochemical oxidants. The aerosols formed during the chemical reactions that create smog caused a marked reduction in visibility and regional haze, and give the atmosphere a brownish cast. Regional haze occurs at distances where the plume has become evenly dispersed into the atmosphere, such that there is no definable plume. The primary cause of regional haze are sulfates and nitrates (primarily as ammonium salts), which are formed from SO2 and NOx through chemical reactions in the atmosphere (Figure 5.2).
Eutrophication: A Widespread Ecological Effect
Eutrophication is a natural process of aging of a body of water. It is a result of a very slow process of natural sedimentation of microscopic organisms which takes geologic times to complete. The completion of the process results in the extinction of the water body.
The process of eutrophication is propelled by increasing concentrations of nutrients necessary for biological activity. First, envision clean and clear water. In this condition, since the nutrients available are minimal, there is no significant biological activity in the water column that can support sedimentation. The water body is healthy and the condition is called oligotrophic. Over time, however, nutrients can build up. A water body with nutrient concentration supporting biological activity that is not objectionable but above that of oligotrophic conditions is considered mesotrophic. In the next stage of the life cycle, the water becomes eutrophic – this is characterized by murky water with an accelerated rate of sedimentation of microorganisms. The final life stage before extinction is a pond, marsh, or swamp.
Although under natural conditions eutrophication occurs very slowly, over geologic times, human activity encourages the production of nutrients and shortens the cycle. People who have a green lawn are prolific producers of nutrients. Farmlands are an excellent source of nutrients. The Chesapeake Bay in Maryland, for example, is loaded with nutrients coming from farmlands as far away as New York.
To survive, microorganisms must be supplied with nutrients containing their components: carbon, hydrogen, oxygen, nitrogen, phosphorus, and trace elements. Since carbon, hydrogen, and oxygen are already in abundance in the environment, eutrophication control focuses on limiting the input of nitrogen and phosphorus. Nitrogen is utilized by organisms in the form of NH4+, NO2−, and NO3−; phosphorus is utilized in the form of orthophosphorus. Algae, the prime cause of eutrophication, can be controlled by limiting their access to nitrogen or phosphorus. Which should be chosen? It has been found that blue‐greens algae, which can survive almost anywhere and can easily outgrow other algae under adverse conditions, can fix nitrogen from the air. For this and other reasons, the default choice, phosphorus control is recommended.
Eutrophication is our final example of the effects included in ecological risk analyses. There are, of course, many others; the literature is ample. Now, however, we are ready to consider another step in an ERA: toxicity testing.
Toxicity Testing
Ecological toxicity tests are classified according to duration (short‐term, intermediate, and/or long‐term), method of adding test solutions (static, recirculation, renewal, or flow‐through), and (to satisfy permit requirements for a proposed pollutant discharge elimination system, and to determine mixing zones, etc.). Detailed contemporary testing protocols are summarized in the regulatory literature (USEPA 1985a, b, 1988, 1989).
Toxicity testing has been widely validated in recent years. Even though organisms vary in sensitivity to effluent toxicity, the EPA has documented that effluent toxicity correlates well with toxicity found in the receiving waters when effluent dilution was measured and that predictions of impacts from both effluent and receiving water toxicity tests compare favorably with ecological community responses in the receiving waters. The EPA has conducted nationwide tests with freshwater, estuarine, and marine ecosystems. Methods include both acute as well as chronic exposures.
Current bioassay methods can assess several different phylogenic groups in four to seven days (USEPA 1988). The tests are based on species of nearly national distribution for which a large body of life history and toxicity sensitivity data are available. Proper testing protocol involves assessment of a range of sensitivities of test species to a particular effluent. Typically, two or three species are considered to eliminate uncertainty for this factor.
Common marine species include Champia parvula, the red alga; Mysidopsis bahia, the mysid shrimp; Menidia beryllina, the inland silversides; and Cyrinidon variegatus, the sheepshead minnow. Common freshwater species include Pimephales promelas, fathead minnow, and Ceriodaphnia dubia, the daphnid shrimp.
At the termination of the test, survival is determined and growth is measured (as an increase in dry weight) compared with control. The acute endpoint is the death of the fish.
Evaluation of Toxicity Test Results
A number of terms are utilized in expressing toxicity test results. Acute toxicity is toxicity severe enough to produce a response rapidly (typically a response observed in 48 or 96 hours). “Acute” does not necessarily imply mortality. In studies of marine organisms, the LC50 is the concentration of effluent in dilution water that causes mortality to 50% of the test population; the EC50 is the effluent concentration that causes a measurable negative effect on 50% of the test population. The NOAEL (no observed acute effect level) is defined as the highest tested effluent concentration that causes 10% or less mortality.
Chronic toxicity is the toxicity impact that lingers or continues for a relatively long period of time, often 1/10 or more of the target organism’s life span. Chronic effects could include mortality, reduced growth, or reduced reproduction. The NOEC (no observable effect concentration) is the highest measured continuous concentration of an effluent or toxicant that causes no observable effect based on the results of chronic testing. The LOEC (lowest observed effect concentration) is defined as the lowest observed concentration having any effect. The LOEC is determined by an analysis of variance techniques.
The use of whole effluent toxicity testing affords a number of advantages. In this approach, the bioavailability of the toxics is measured and the effects of any synergistic interactions are also considered. Because the aggregate toxicity of all components of a wastewater effluent is determined, the toxic effect can be limited by limiting only one parameter, the effluent toxicity. Because contemporary receiving water management strategies are based on site‐specific water quality criteria, toxicity testing facilitates comparison of effluent toxicity with site‐specific water quality criteria designed to protect representative, sensitive species and allow for establishment of discharge limitations that will protect aquatic environments.
Ecological Risk Characterization
Historically, the most common approach to risk characterization was the calculation of hazard quotients. This was adopted from the HHRA field, where this approach is still used. Simply, it compares chemical concentrations in ambient media to some toxicity benchmark. If the quotient exceeds 1, there is a potentially unacceptable risk. This approach has found use in predictive assessments and in screening‐level (otherwise known as preliminary or tier I) retrospective ERAS, where it is used to refine the list of contaminants of concern and to focus a subsequent, more detailed assessment. However, for a baseline ERA, the quotient method should be used with caution. It is especially important to realize that the magnitude of the excess in the hazard quotient has no quantitative relation to the magnitude of potential toxic effects. Calculating several hazard quotients using different benchmarks (e.g. derived from different toxicity data, such as acute, chronic, or population level effects) has more direct applicability than using a single benchmark.
Because ecological effects can be measured in a retrospective ERA, an epidemiological, weight‐of‐evidence approach can be used. This approach depends upon weighing multiple lines of evidence, such as those provided by the field surveys, toxicity tests, and ambient media chemical analyses and literature toxicity data. Risk assessors, risk managers, and the public will have more confidence in a risk assessment that uses the weight‐of‐evidence approach, because it integrates all sources of information, attempts to reconcile conflicting data, and can account for the bioavailable fraction of chemicals in the environment, and the effects of multiple contaminants.
The primary line of evidence in the weight‐of‐evidence approach is the field survey data. Field surveys monitor actual ecological impacts, and therefore are the most credible line of evidence. However, as discussed in Section 5.3.4.1, field surveys have their limitations. Also, many ERAs will not have the budget necessary to conduct field surveys, and nocturnal, migratory, secretive, or wide‐ranging species are not easily surveyed. Also, small impacts are not readily apparent in field surveys. Therefore, other lines of evidence are used as support.
Toxicity tests give an indication of whether ambient media are toxic. When several contaminants exceed benchmarks and there is an impact in the toxicity tests or field surveys, it is important and necessary to evaluate the magnitude of the effect caused by the contaminants which exceeded benchmarks. Using media contaminant analysis and the information provided in the toxicity profile, compiled in accordance with Section 5.3.5, an evaluation is conducted of which contaminants could be responsible for the observed toxicity. Combining all of these lines of evidence will present a picture of actual or potential impacts at the site and contaminants responsible for the impacts. In some cases, benchmarks may indicate unacceptable risk while field observations show no measurable impacts. Therefore, the weight of evidence suggests no unacceptable risks to a community, even though contaminant concentrations exceeded benchmarks. Reconciling multiple lines of evidence is difficult and requires experience and understanding of the ecosystem being evaluated.
Leave a Reply