The process is also known as supercritical water oxidation. This is a novel technology to produce methane‐rich synthesis gas as an alternative to replace the conventional recovery system (Naqvi et al. 2010). Heat demand for bringing water to supercritical conditions is less than that for evaporating at subcritical pressure. This phenomena leads to better energy savings as compared to conventional recovery system (Sricharoenchaikul 2009; Vogel et al. 2005). High‐water content in the black liquor – nearly 80% under supercritical condition – would increase gasification reactions that lead to high organics conversion to the synthesis gas (Calzavara et al. 2005; Williams and Onwudili 2006). This phenomenon results in direct introduction of black liquor to hydrothermal gasifier, removing energy demanding evaporation unit in conventional process, thus decreasing the overall stream demand of the pulp mill. The multi‐effect evaporation unit represents nearly 37% of total heat demand of the pulp mill and can be removed in this technique. Unlike other gasification processes, black liquor from the digestion unit is directly introduced to catalytic hydrothermal gasifier at supercritical water conditions (600 °C, 300 bar). The catalytic hydrothermal gasification process involves three steps:
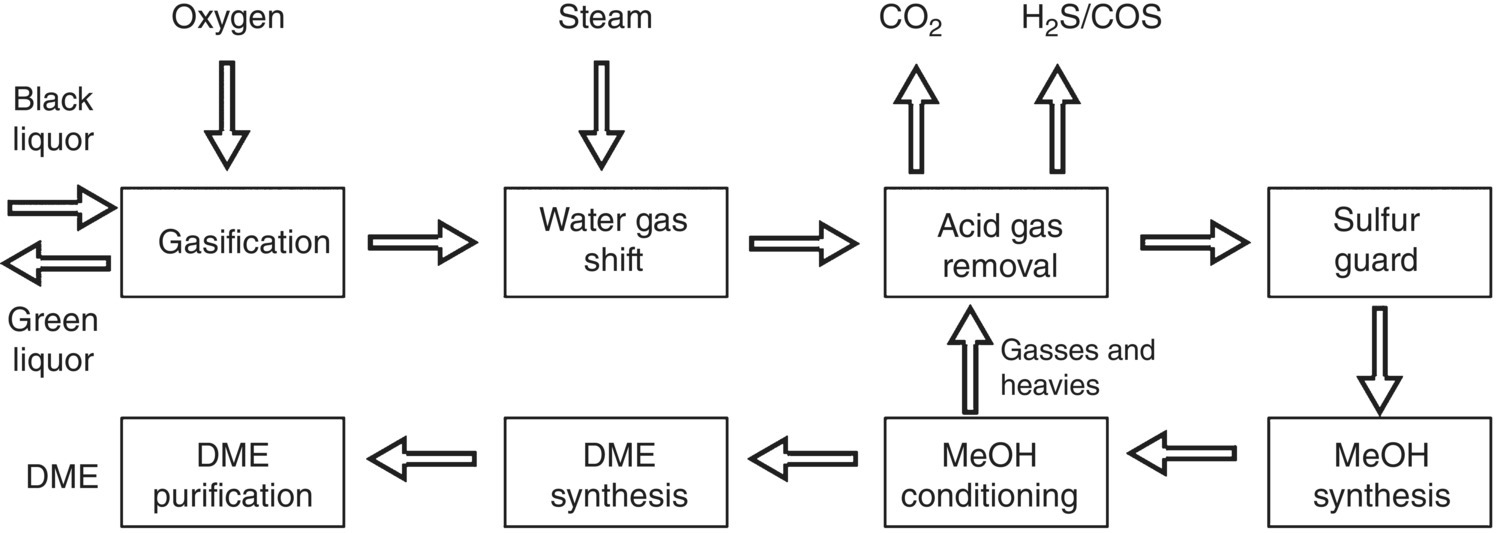
- Heat up phase (decomposition unit): During this phase, large molecules in black liquor hydrolyze to form alcohols due to the presence of lignin. Cellulose present in lignin decomposes rapidly in water at about 250 °C.
- Salt separation: In salt separator, inorganic salts present in black liquor precipitate and are returned to the pulp mill.
- Catalytic reactor: This is the actual methane synthesis unit where smaller organic molecules, such as carboxylic acid, alcohols, and aldehydes, are converted to CH4, CO2, H2, and CO.
Carboxylic acid is converted to methane, carbon dioxide, and hydrogen. The tar formation is avoided due to supercritical conditions and presence of catalyst.
Sricharoenchaikul (2009) studied the feasibility of supercritical water oxidation technique to convert black liquor to value‐added fuel products and recovery of pulping chemicals. The experiments were conducted in a quartz capillary heated in a fluidized bed reactor based on important operating parameters which were pressure, temperature, feed concentration, and reaction time. The investigator found that pressure between 220 and 400 bar had insignificant influence on the syngas and carbon conversion. But increasing temperature and residence time between 375–650 °C and 5–120 seconds resulted in high‐carbon conversion, greater synthesis gas production, and energy efficiency.
Fischer–Tropsch Liquids
Commercial Fischer–Tropsch catalysts include iron‐ and cobalt‐based materials. Cobalt catalysts produce a large heavy‐wax fraction that can be easily and with high selectivity refined into desired lighter products by subsequent hydrocracking (breaking up of the large hydrocarbon molecules into desired final products in a hydrogen‐rich environment). Hydrocracking of the large straight‐chain hydrocarbons formed by FT synthesis can be done under much less severe temperature conditions (350–400 °C for cracking to C5–C18 range hydrocarbons) than is required for hydrocracking of aromatic molecules found in conventional petrochemical refining. Iron‐based catalysts produce a broader product mix that requires a greater level of refining than with cobalt catalysts. Also, unlike cobalt catalysts, iron catalysts promote water‐gas shift activity (CO + H2O → H2 + CO2), making them well suited for use with syngas characterized by H2/CO ratios below the stoichiometric value of 2.2 for FT synthesis.
Leave a Reply