The AC is a carbonaceous material produced by steam activation (at ~900 °C). It has high mechanical strength against abrasion and crushing. Its surface area is 150–300 m2/g, less than the conventional activated carbon, but not much higher than the metallurgical coal. AC comes in tablet or almond‐type shape and is generally much larger than the activated carbon. AC is easier to produce and less expensive than activated carbon. Figure 9.15 shows the internal model of AC and its pore structure. Figure 9.16 shows mercury vapor removal efficiency versus time.
Thermal Regeneration of AC
At the temperatures in the regenerator heating section adsorbed sulfuric acid, ammonium sulfate, and ammonium bisulfate are decomposed to SO2. AC pellets enter via lock hoppers and is in gravity counter‐flow against desorbed gases and N2 carrier gas. Sulfur‐rich gas containing SO2, CO2, N2, and H2O exits the regenerator to an acid plant as follows:
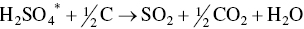
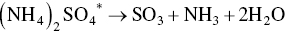
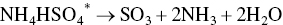
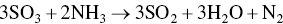
(*denotes adsorbed species; non‐isothermal desorption kinetics reach peak rates at 300 °C and completion by 450 °C).
Performance and Benefits of ReACTTM
Table 9.3 presents a typical performance at J‐Power EnTech 2x600MW ISOGO coal‐fired power plant in Japan (Reynolds, personal communication). The benefits of the advanced multi‐pollutants control by the ReACT™ can be summarized as follows:
- By‐product revenue – Sulfuric acid is the world’s number one commodity chemical with a market value of $50 200/T. Instead of producing disposal gypsum or fly ash/gypsum waste, ReACT™ produces saleable by‐product.
- Avoided disposal costs – For every ton of SO2 controlled in conventional flue‐gas desulfurization (FGD), about three tons of solid waste is generated. More if fly ash is part of the FGD waste stream.
- Near zero water use – ReACT™ uses minimal water, in significant contrast to FGD systems. For a 500 MW plant, a WFGD (wet flue gas desulfurization) system would require 275 000 000 gal/year while ReACT™ would use near zero.
 Figure 9.15 Internal model of AC – pore structure.
Figure 9.15 Internal model of AC – pore structure. Figure 9.16 Mercury vapor removal efficiency (%) versus time.Table 9.3 Typical performance of advanced multi‐pollutant removal.PollutantsEmissions permitOperating resultsEfficiency (%)Inlet concentrationOutlet concentrationSOx10 ppm
Figure 9.16 Mercury vapor removal efficiency (%) versus time.Table 9.3 Typical performance of advanced multi‐pollutant removal.PollutantsEmissions permitOperating resultsEfficiency (%)Inlet concentrationOutlet concentrationSOx10 ppm
(0.025 lb/MMbtu)>98<410 ppm
<0.85 lb/MMbtu<1 ppm
<0.002 lb/MMBtuNOx13 ppm
(0.02 lb/MMbtu)10–50<20 ppm
0.03 lb/MMbtu<7 ppm
<0.011 lb/MMbtuParticulate5 mg/Nm3
(0.004 lb/MMbtu)>95
(>99.9 w/ESPs)<100 mg/Nm3
<0.1 lb/MMbtu<3 mg/Nm3
<0.002 lb/MMbtu
(w/backend ESP)Hg––>902.5 μg/Nm3<0.25 μg/Nm3 - Minimal plant modifications – ReACT™ can flow to the existing stacks with no change in liner materials. ReACT™ is located downstream of existing equipment and does not necessitate modifications to upstream equipment.
- NOxperformance options – ReACT™ systems may be designed for a range of NOx reduction options – from co‐benefit levels of 30% through alternative designs reaching 80%.
Leave a Reply